Introduction: Burkitt lymphoma (BL) is the commonest childhood cancer diagnosed in Sub-Saharan Africa (SSA). It is highly curable with survival rates >90% in resource-rich settings with dose-intensive regimens that contain the anti-CD20 monoclonal antibody, rituximab, high-dose methotrexate (HDMTX; ≥ 3000 mg/m 2) and high-dose cytarabine. In contrast, outcomes in SSA where patients typically receive less intense chemotherapy with lower-dose methotrexate (≤1000 mg/m 2) and without rituximab, remain poor. Major drawbacks to implementing HDMTX regimens in SSA include lack of adequate supportive care and inability to monitor drug levels. Improving long-term outcomes for BL in SSA requires graduated, stepwise, and cautious escalation of treatment tailored to available supportive care infrastructure. Based on the available evidence, we adopted a resource-adapted HDMTX-containing regimen as our standard of care for pediatric BL in Lilongwe, Malawi. We report preliminary data on patients treated with this regimen.
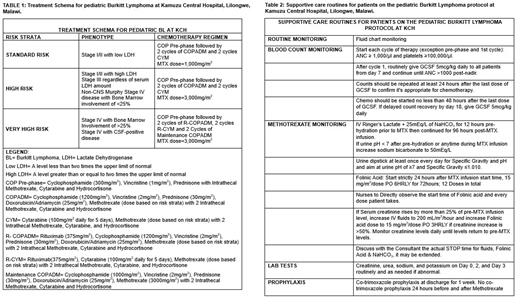
Methods: This is a prospective study of children (<18 years) with newly diagnosed BL, treated with our locally adapted regimen at Kamuzu Central Hospital in Malawi. This risk-stratified regimen incorporates HDMTX at doses of 1000-3000 mg/m 2 per cycle (Table 1) and is based on the Group B arm of the FAB/LMB backbone (standard of care in high-resource settings) with a 60% dose reduction of anthracycline-based on previously published data. Rituximab and two maintenance cycles were incorporated into the therapy for very high-risk patients (Table 1). Treatments were administered in 14-day cycles. Patients not in complete remission (CR) at the end of the first consolidation cycle received 4 cycles of DHAP (Dexamethasone, Cisplatin, HD-Cytarabine, Prednisone). To mitigate toxicity, we established locally appropriate best practices for supportive care and extended bicarbonate-containing fluid hydration for days with rate adjustments based on changes in daily creatinine levels in lieu of MTX levels-which were unavailable (Table 2). Adverse events (AE) were defined by Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 criteria. Using descriptive statistics, we report on the frequency of severe AEs in this cohort.
Results: Between June 2022 and July 2023, 40 consecutive patients with biopsy-proven BL were treated with this approach. They were mostly male (n=26, 65%) with a median age of 9 years (interquartile range, 6-12). The majority (n=22, 55%) had Murphy stage III disease, and 15 (38%) were stage IV at diagnosis. Five patients (13%) had central nervous system (CNS) disease, and 12 (31%) with bone marrow (BM) disease. All patients were stratified as high-risk or very high-risk. Nineteen (48%) have completed their planned first-line therapy, 7 (17%) are currently in therapy, 8 (20%) died before treatment completion, and 6 (15%) abandoned therapy. Treatment delays occurred in 12/107 (11%) HDMTX-containing cycles. For the non-rituximab HDMTX cycles (n=90), there have been 14 episodes (16%) of grade ≥3 febrile neutropenia, 1 episode (1%) of grade ≥3 mucositis, and 5 episodes (6%) of grade ≥3 anemia. One patient developed grade 3 acute kidney injury, which resolved with hydration. In patients who received rituximab (n=17) there have been 3 episodes (18%) of grade ≥3 febrile neutropenia, and 1 episode (6%) of grade ≥3 mucositis or anemia. In total, there were 12 (30%) deaths: 5 disease-related deaths, 4 early deaths from sepsis, or tumor lysis before pre-HDMTX, 1 unknown cause, and 2 (6%) treatment-related deaths associated with HDMTX. Twenty-three patients had interim assessment at the end of the first consolidation and 18 (78%) were in CR. Of the patients with relapse or progressive disease (n=8), 4 (50%) had either CNS or BM disease.
Conclusion:
We demonstrate feasibility of administering dose-intensive intermediate dose MTX-containing treatment for pediatric BL in a very resource-limited setting with rigorous surveillance and supportive care. We will build on this by incorporating rituximab into all risk-groups, while evaluating its impact on efficacy and the immune reconstitution in a population with high prevalence of malnutrition, malaria, and tropical infections. Furthermore, given higher treatment-failure rates in patients with CNS or BM disease, strategies to intensify treatment in this cohort in the context of available support care resources will be needed.
Disclosures
Allen:Sobi, Inc: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal